Predicting Stable Anions and Cations
Main group elements (Group A) will gain or lose minimum number of electrons to have a filled shell (noble gas configuration).
Example:
Na 1s2 2s2 2p6 3s1 → Na+ 1s2 2s2 2p6 + e-
Cl 1s2 2s2 2p6 3s2 3p5 + e- → Cl- 1s2 2s2 2p6 3s2 3p6
Transition group elements (Group B) generally lose outer shell s - electrons. Many also lose electrons to have either a filled or half-filled subshell.
Example:
Cu [Ar] 4s2 3d9 → Cu2+ [Ar] 3d9 + 2 e-
The Cu2+ lost its outer shell s electrons.Fe [Ar] 4s2 3d6 → Fe3+ [Ar] 3d5 + 3 e-
The Fe3+ has a half-filled 3d subshell.
Ionic sizes
A cation is smaller than its parent atom. An anion is larger than its parent atom.
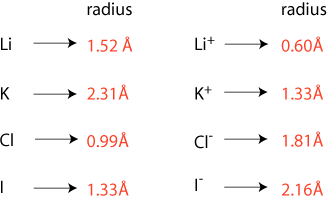
Generally, ion size increases going down a group. Change in ion size horizontally is complicated because we change from cations on the left to anions on the right. However, it is helpful to look at the relative size of isoelectronic ions.
Isoelectronic Ions-- ions containing the same number of electrons.For example, O2-, F-, Na+, Mg2+, and Al3+ all have the electron configuration of Neon. But the charge of the nucleus increases from +8 on Oxygen to +13 for Aluminum. So the same number of electrons is Aluminum (Al3+) will be bound much closer to the nucleus than the electrons of O2-.
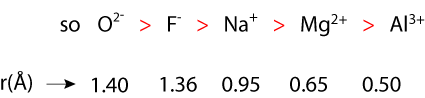
For a series of isoelectronic ions, the size decreases as the nuclear charge, z, increases.
Arrange the following ions in order of increasing size: Se2-, Br-, Rb+, Sr2+
| smallest | Sr2+ | < | Rb+ | < | Br- | < | Se2- | largest |
| 38 protons | 37 protons | 35 protons | 34 protons | |||||
| 36 electrons | 36 electrons | 36 electrons | 36 electrons |
Big Picture: Chemistry is all about transferring electrons between different atoms and/or molecules. The different chemical properties of each atom in the periodic table is a result of the different degrees of which each atom wants to gain or lose electrons.
Homework from Chemisty, The Central Science, 10th Ed.
7.5, 7.13, 7.15, 7.17, 7.19, 7.21, 7.23, 7.25, 7.27, 7.29, 7.31, 7.33, 7.35, 7.37, 7.39, 7.41, 7.43, 7.45, 7.47, 7.49, 7.51